Phase II trial publication
Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial
Summary
The ChAdOx1 nCoV-19 coronavirus vaccine, developed by teams at the University of Oxford, has been shown to trigger a robust immune response in healthy adults aged 56-69 and those over 70 years of age. The data, published today in The Lancet, suggest that one of the groups most vulnerable to serious illness, and death from COVID-19, could build immunity.
Older adults have been shown to be at higher risk from COVID-19 and should be considered to be a priority for immunisation should any effective vaccine be developed for the disease. Reporting on data from a Phase II trial of the ChAdOx1 nCoV-19 vaccine, the study shows that volunteers in the trial demonstrate similar neutralising antibody titres, and T cell responses across all three age groups (18-55, 56-79, and 70+).
These data are consistent with the Phase I data reported for healthy adults aged 18-55 early this year.
Aims
The outcomes reported at this stage of the study include the safety of the vaccine and the immune responses of participants in different age groups following vaccination. It is important to assess how well the vaccine works in older people, as these age groups are more severely affected by COVID-19 disease. Sometimes vaccines are less effective in older people, so it is important to find out at an early stage how well the immune system responds to the vaccine in those over the age of 55.
The main objectives of the trial, including whether the vaccine can prevent COVID-19 disease will be reported at a later date.
The Trial
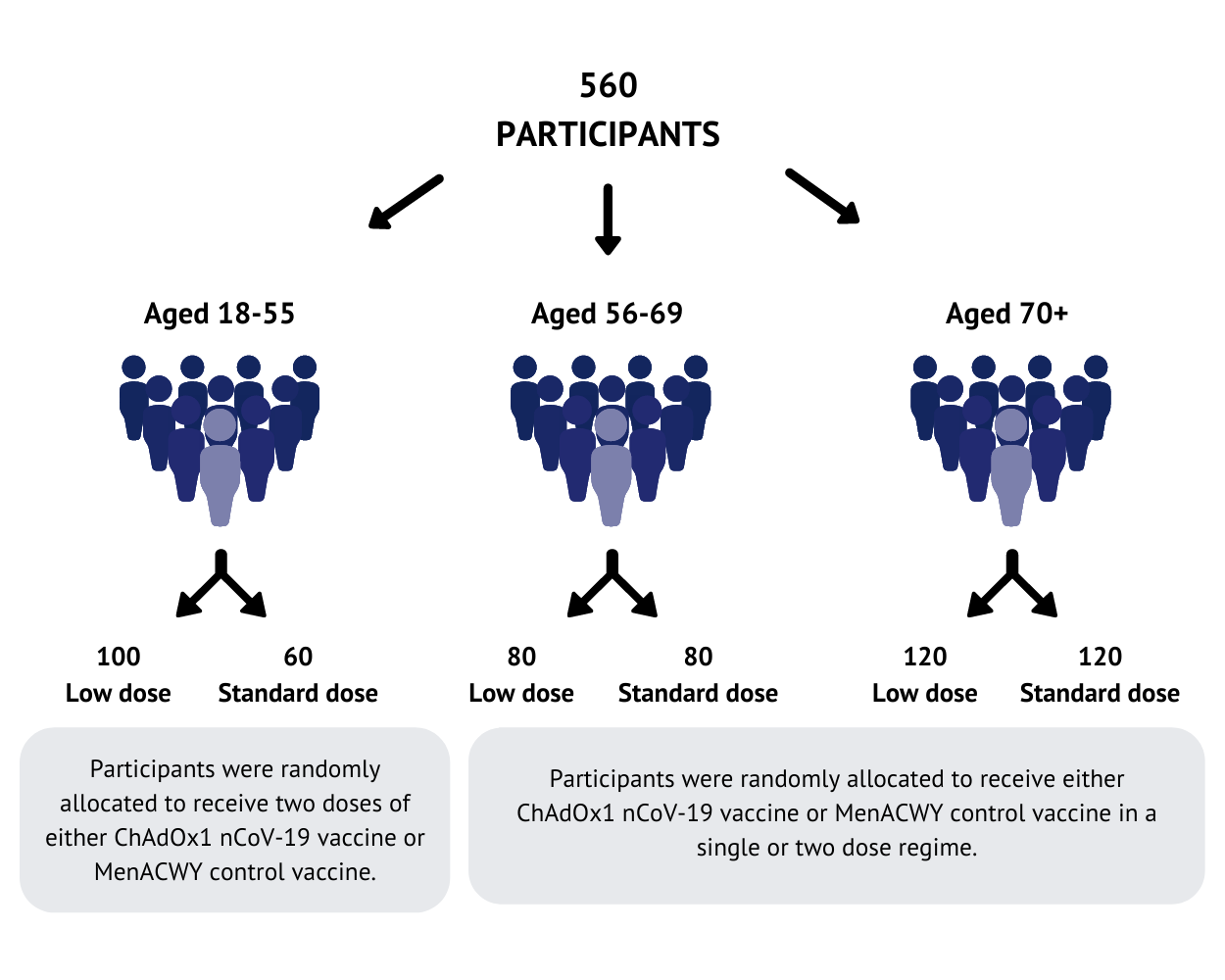
560 participants have been recruited at Oxford and Southampton study sites. Participants were randomly allocated to receive either the ChAdOx1 nCoV-19 vaccine or a licensed vaccine (MenACWY) that is used as a ‘control’ for comparison. In the 18-55 age group, half received the ChAdOx1 nCoV-19 vaccine and half received the control. For the older age groups (56-69 and over 70), more people received the ChAdOx1 nCoV-19 vaccine than the control vaccine.
Two doses were tested as part of this study: a standard dose (the same dose used in the Phase I study), and a lower dose, tested before the standard dose in the older age groups. The doses used in this trial were chosen based on previous experiences with other ChAdOx1 based vaccines. All the participants were healthy adults with no severe health conditions.
After receiving the vaccine, participants recorded any symptoms experienced for 7 days. Following the vaccination visit, participants attended a series of follow-up visits to check their observations and take blood samples. These blood samples were used to assess the immune response to the vaccine, by measuring neutralising antibody and T cell responses.
Study participants will not know whether they received the ChAdOx1 nCoV-19 vaccine or the control vaccine until the end of the trial.
The Results:
Safety
There were no safety concerns associated with the vaccine and no unexpected symptoms shown in those participants who received it. The safety profile was similar to that seen in the Phase I study and in other similar vaccines, including pain and tenderness at the injection site, and flu-like symptoms such as headache, fever and muscle aches. Fewer adverse events were reported in the older age groups than in the 18-55 year olds; adverse reactions reduced as age increased. Adverse events were also less common after the booster dose compared with the initial vaccination. As we compared two different doses in this study, we also saw that there were fewer adverse reactions in those who received the lower dose of the vaccine. No severe adverse events related to the ChAdOx1 nCoV-19 vaccine were reported.
Immunogenicity – Antibodies
Antibodies play an important role in the immune response to viruses. This study assessed both the quantity and quality of antibody found in participant blood samples. An ELISA (enzyme-linked immunosorbent assay - a plate-based laboratory technique) was used to detect and measure the quantity of antibodies in the blood that recognise the spike protein.
In our previous study, we reported that following a single vaccination all participants produced spike specific antibody by 14 days, with the antibody response peaking at 28 days. This response was maintained for up to 56 days post vaccination, showing potential for long term maintenance of the antibody response. In participants who received a second boost vaccination at 28 days, there was a further increase in the peak response. In this study, we showed that antibody responses in the older age groups (both 56-69 and over 70) were comparable to those seen in younger adults. Similarly, after a second dose of the vaccine, antibody levels increased, and this was also consistent for the older age groups.
In addition to measuring the quantity of antibody, the study also examined the quality of antibody, its ability to neutralise the virus. Like the previous study, good levels of neutralising antibodies were produced, and this was consistent across all age groups.
As we are still conducting long term follow up of participants in the study, data is not yet available on how long antibody lasts, however previous data using the same ChAdOx1 vaccine platform suggest this should be long lived (more than 1 year).
Immunogenicity – T Cells
T cells play an important role in the immune response to viral infections. Some T cells are responsible for killing viruses inside infected cells, whilst others are responsible for providing help to other components of the immune response. There is increasing evidence that T cells play an important role in preventing serious disease with natural infection of the COVID-19 virus.
T cell numbers were measured using the ELISpot assay (enzyme-linked immune absorbent spot - an assay that detects the proportion of T cells specifically stimulated by the vaccine). In our previous paper, we showed that following vaccination, the number of T cells in the blood of participants increased and peaked at 14 days post vaccination. Despite a dip afterwards, this response was well maintained up to a month after. In this study, we showed that after a single vaccination, T cell responses were highly comparable in all ages and across different doses. In both studies, a second vaccination does not further increase the T cell response.
What’s next?
The participants in this study continue to be monitored to assess how well the immune responses are maintained over a longer time period.
If participants develop COVID-19 symptoms during the study, they can contact a member of the clinical team, to check whether they have become infected with the virus.
To assess whether the vaccine works to protect from COVID-19, the statisticians in our team will compare the number of infections in the control group with the number of infections in the vaccinated group. How quickly we reach the numbers required will depend on the levels of virus transmission in the community. With the current low transmission levels in the UK, this could take many months.
We have completed recruitment of over 10,000 people to our Phase II/III study which aims to assess the efficacy of the vaccine, and to compare the different dose schedules. To increase the chance of achieving an efficacy result sooner, we have prioritised those who have a higher chance of being exposed to the SARS-CoV-2 virus, such as frontline healthcare workers, frontline support staff and public-facing key workers. Conducting studies in multiple locations where transmission levels vary also increases the chances of reaching an efficacy endpoint sooner.
The full publication is available here:
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32466-1/fulltext




